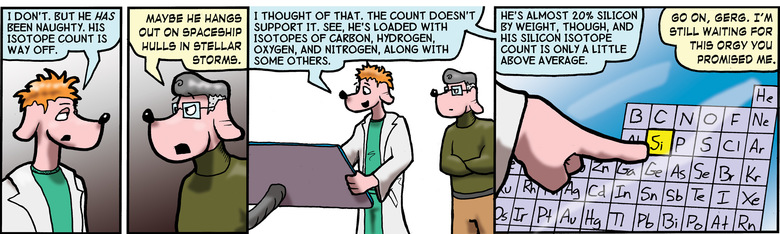
The "identity" of an element is determined by the number of protons it has. Carbon, Nitrogen, and Oxygen have 6, 7, and 8 respectively. Silicon has 14.
The "isotope" of an element is determined by the number of neutrons it has. The predominant isotope of carbon has 6. Carbon 14, for instance, has eight, and is unstable, decaying into Nitrogen 14 by shedding a beta particle. This rate of decay is known, which allows for the "Carbon-dating" procedure we've all heard so much about.
The statements above have been grossly over-simplified.
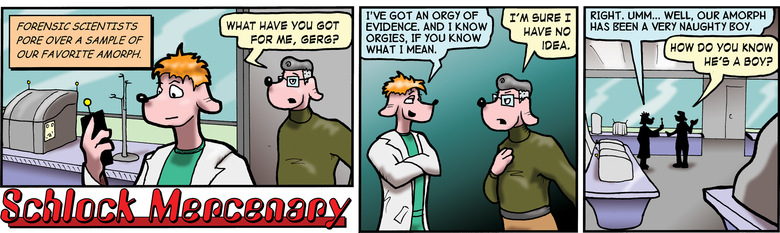
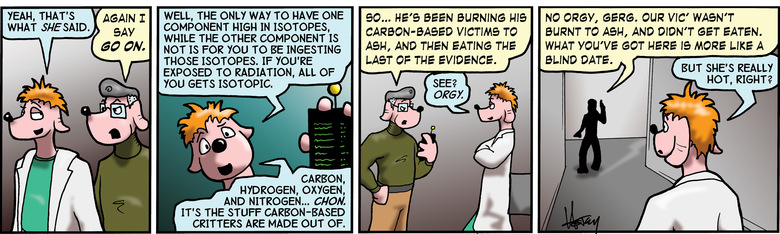
The gross over-simplification above is nothing compared to the oversimplification in the strip. The strip has been oversimplified to the point that it's almost certainly WRONG. The radioisotopes you'll find if you bombard CHON with a stream of high-energy protons will be all over the table. For purposes of ACCURATE simplicity, however, it IS possible to look at a mixture of stuff, count isotopes, and determine whether it is naturally occurring, or whether it was created with the help of a particle accelerator like a plasma cannon. It's also possible to determine which of the elements in that mixture were in front of the accelerator, and which were added after the fact.
The author briefly toyed with the idea of mapping dozens of radioisotopes into Gerg's dialog, and ran out of time, patience, and space simultaneously. Thus, in the spirit of being entertaining first, and accurate SECOND, Gerg's dialog was run in its current form.
This simplification would have been neither necessary nor acceptable in a television drama about forensic science. Never!